how do leucine zippers work
The slider body that separates and joins the teeth together. These amino acids are spaced out in each regions polypeptide sequence in such a way that when the sequence is coiled in a 3D alpha-helix the leucine.

Pdf Leucine Zipper Semantic Scholar
Toshio HakoshimaNara Institute of Science and Technology Nara Japan The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position and forms an a-helical conformation which facilitates dimerization and in somecaseshigheroligomerizationofproteins Inmanyeukaryoticgeneregulatoryproteins the ZIP.

. The leucine zipper is a fashioned third-dimensional structural motif in proteins and it has that identify because leucines arise each seven amino acids. DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor. Gcn4 Basic Region Leucine Zipper Complex With Ap-1 DNA.
The top and bottom stop which prevents the zipper from coming apart at each end. The leucine zipper is formed by amphipathic interaction between two ZIP domains. HTLV-1 basic leucine zipper factor downregulates cyclin D1 expression via interactions with NF-κB.
BZIP basic leucine zipper transcription factors as one of the largest transcription factor regulatory families play very important roles in responses to these abiotic stresses. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an αhelical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving. The HTLV-1 basic leucine zipper bZIP factor HBZ which is encoded by the minus-strand of the provirus is expressed in all cases of ATL and involved in T cell prol.
The amino acid side chain in position d is oriented away from the central axis and toward the aqueous phase. How do leucine zippers work Tuesday March 15 2022 Edit Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. My Masters Work -.
Leucine side chains in position a face each other across the central axis of the coiled coil and handshaking is easy. The leucine zipper is an amphipathic a helix containing heptad repeats of Leu residues on one face of the helix and serves as a dimerization module. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an αhelical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving.
Nature - Action of leucine zippers. Genetic physical and structural studies of the leucine zipper identify interactions that help determine the stability and specificity of dimerization and DNA binding. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an α-helical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving.
The pull tab that allows you to use the slider. These can form homodimers and heterodimers through their leucine-zipper domains. The tape which is the fabric to which the teeth are attached.
The track which consists of teeth or coils that interlock. Of active oxygen species is a type of stress called photo. Abstract In the basic-region leucine-zipper domain flexible DNA-binding arms are juxtaposed by a two-stranded parallel coiled-coil motif called the leucine zipper.
Elements of a zipper include. On dimerization the leucine-zipper a helices form a parallel-coiled coil based on hydrophobic interfacial side-chain packing 55. The ZIP domain is found in the alpha-helix of each monomer and contains leucines or leucine-like amino acids.
These responses are usually originated by regulating the expression of relevant genes. The leucine zipper is a dimeric parallel coiled-coil but amphipathic helices can also oligomerize to form parallel coiled-coils that are trimers tetramers or pentamersThe majority of B-ZIP leucine zippers contain valines in the a positionandleucinesinthedpositionKimandcolleagues changed both of these amino acids to isoleucine which. Spacefill of Leu -67 and Leu -74 sidechains.
BZIP TFs could be activated by drought high salt and chilling damages.

X Ray Structure Of Gcn4 B Zip Motif Bound To A Tre Dna Sequence Download Scientific Diagram
Fosb Jund Bound To Cognate Dna A Bzip Domain With Leucine Zipper And Download Scientific Diagram

Modelling Of The Interaction Of The Atbzip63 Helical Download Scientific Diagram

File Creb Protein Png Wikipedia

Buried Asparagines Determine The Dimerization Specificities Of Leucine Zipper Mutants Pnas

By511 Lecture 21 Leucine Zipper Protein Motif Flashcards Quizlet

Molecular Basis Of The Out Of Register Leucine Zipper Assembly In Mitf Download Scientific Diagram

Attractive Interhelical Electrostatic Interactions In The Proline And Acidic Rich Region Par Leucine Zipper Subfamily Preclude Heterodimerization With Other Basic Leucine Zipper Subfamilies Journal Of Biological Chemistry
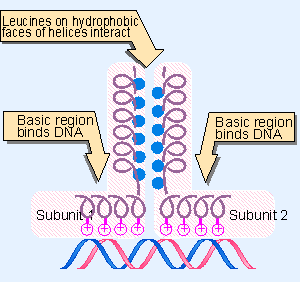
8 Leucine Zippers Are Involved In Dimer Formation Genes Vii

Pdf Leucine Zipper Semantic Scholar
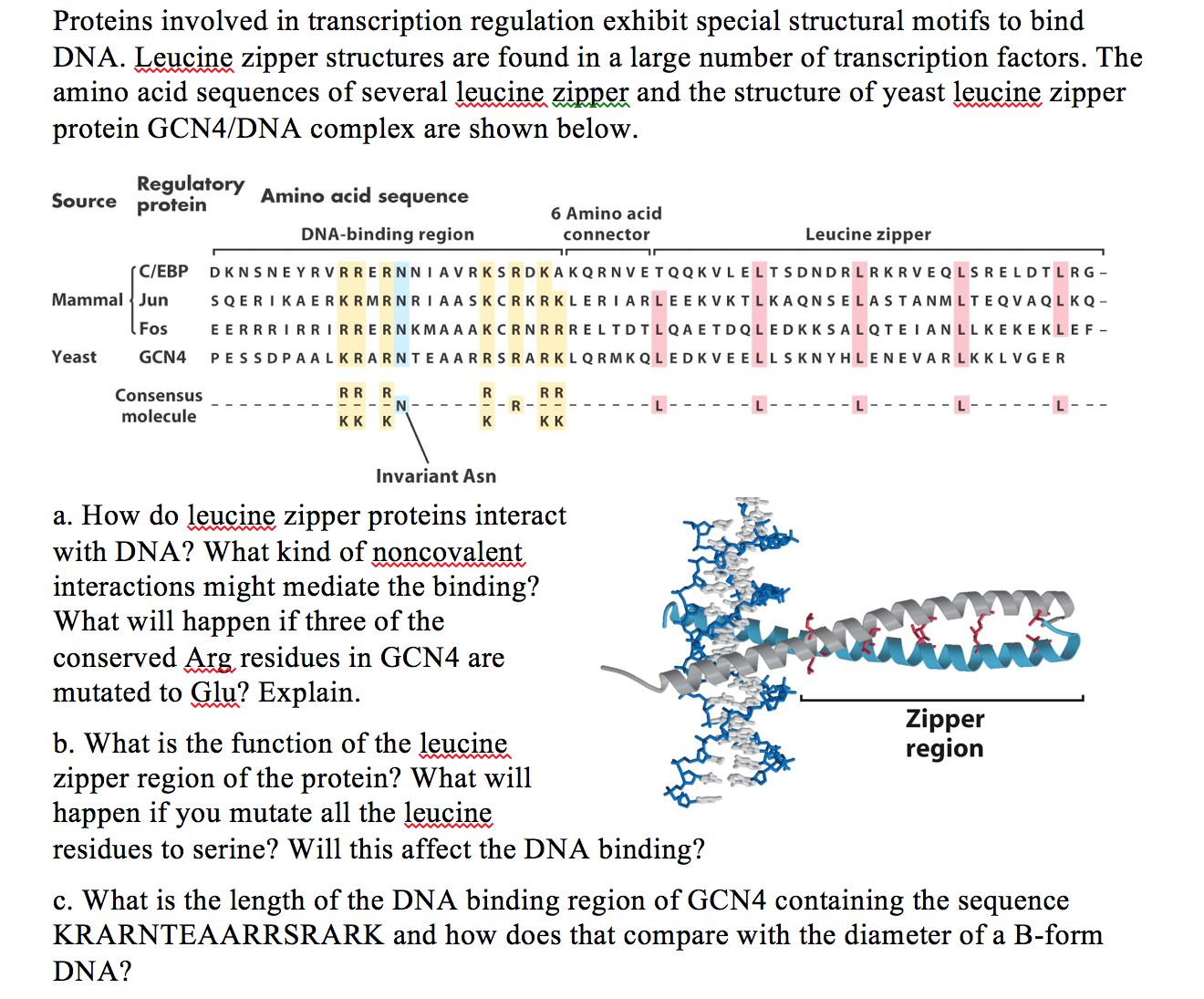
Solved Proteins Involved In Transcription Regulation Exhibit Chegg Com

Principle Of The Universal Peptide Break Technology And Leucine Zipper Download Scientific Diagram
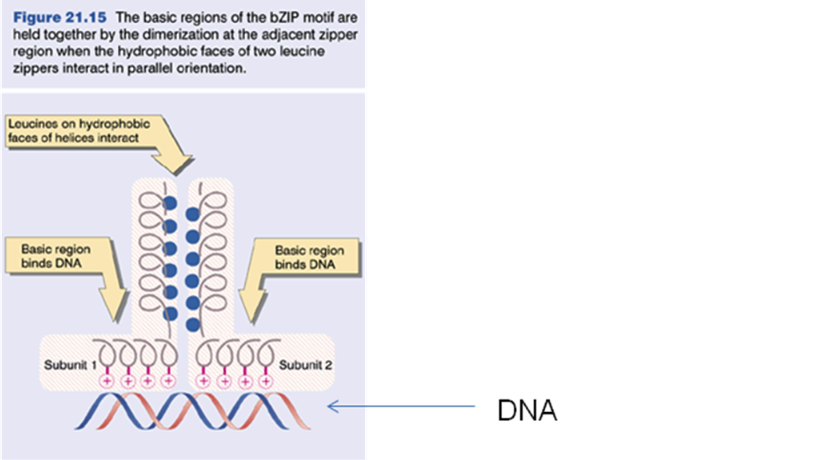
Solved Figure 21 15 The Basic Regions Of The Bzip Motif Are Chegg Com

Basic Leucine Zipper Transcription Factor An Overview Sciencedirect Topics

Leucine Zippers Leucine Zipper Of The Yeast Activator Protein Gcn4 Download Scientific Diagram

Chapter 13 Transcriptional Control And Epigenetics Chemistry


